Chemistry Question
Moderator: Community Team
Forum rules
Please read the Community Guidelines before posting.
Please read the Community Guidelines before posting.
Chemistry Question
Is a diamond one single molecule?
I do it because I can
I can because I want to
I want to because you said I couldn't
I can because I want to
I want to because you said I couldn't
Re: Chemistry Question
I don't see why not... atoms held together by covalent bonds... sounds like a molecule to me. 
natty_dread wrote:Do ponies have sex?
(proud member of the Occasionally Wrongly Banned)Army of GOD wrote:the term heterosexual is offensive. I prefer to be called "normal"
Re: Chemistry Question
no, its not.
that would be like saying the ocean is one molecule.
that would be like saying the ocean is one molecule.
-
Strife
- Posts: 2668
- Joined: Fri Jul 20, 2007 3:24 pm
- Gender: Female
- Location: Now something has kept me here too long.
Re: Chemistry Question
It's several chains of molecules linked together.
Re: Chemistry Question
The molecules of water aren't linked though. It's just a bunch of H20's, with some NaCl and more contaminants.jonka wrote:no, its not.
that would be like saying the ocean is one molecule.
In a pure diamond?Strife wrote:It's several chains of molecules linked together.
natty_dread wrote:Do ponies have sex?
(proud member of the Occasionally Wrongly Banned)Army of GOD wrote:the term heterosexual is offensive. I prefer to be called "normal"
Re: Chemistry Question
John is right, the carbon atoms are all linked together by covalent bonds (one carbon atom is bonded to four others).john9blue wrote:In a pure diamond?Strife wrote:It's several chains of molecules linked together.
I do it because I can
I can because I want to
I want to because you said I couldn't
I can because I want to
I want to because you said I couldn't
-
Strife
- Posts: 2668
- Joined: Fri Jul 20, 2007 3:24 pm
- Gender: Female
- Location: Now something has kept me here too long.
Re: Chemistry Question
There's no such thing as a "pure diamond"... it's just multiple carbon atoms, combined into carbon molecules, compressed and superheated and several molecules of such combined into chains which make the near indestructible lattice structure of the diamond.LYR wrote:John is right, the carbon atoms are all linked together by covalent bonds (one carbon atom is bonded to four others).john9blue wrote:In a pure diamond?Strife wrote:It's several chains of molecules linked together.
A diamond is just several atoms of Carbon-14, each one combines with four other Carbon atoms to make a molecule. Thus, several molecules... otherwise you're an idiot to think a single molecule can be visible to the naked eye(Exception of DNA or something as such).
- Balsiefen
- Posts: 2299
- Joined: Wed Aug 30, 2006 6:15 am
- Gender: Male
- Location: The Ford of the Aldar in the East of the Kingdom of Lindissi
- Contact:
Re: Chemistry Question
It's a giant covalent structure. This, though there is a continuous link between all atoms in it's makeup, is rarely described as a molecule.
Re: Chemistry Question
a diamond is carbon that has been under alot of pressure
since i'm carbon does this make me a molecule
since i'm carbon does this make me a molecule
- jonesthecurl
- Posts: 4625
- Joined: Sun Mar 16, 2008 9:42 am
- Gender: Male
- Location: disused action figure warehouse
- Contact:
Re: Chemistry Question
No, but if the bike in your avi was coal, you'd soon have a diamond.
instagram.com/garethjohnjoneswrites
Re: Chemistry Question
jonesthecurl wrote:No, but if the bike in your avi was coal, you'd soon have a diamond.
Re: Chemistry Question
your wit kills mejonesthecurl wrote:No, but if the bike in your avi was coal, you'd soon have a diamond.
Re: Chemistry Question
Superheated? That is not how natural diamonds are formed...Strife wrote:There's no such thing as a "pure diamond"... it's just multiple carbon atoms, combined into carbon molecules, compressed and superheated
Since when has a diamond been near indestructible? If I dropped it off of a skyscraper it would shatter into a million pieces. Hard, yes, but indestructible... no.Strife wrote:and several molecules of such combined into chains which make the near indestructible lattice structure of the diamond.
No, a diamond if comprised of much more than "several atoms..."Strife wrote:A diamond is just several atoms
They do not combine with each other, they all share electrons with each other, which is why it can be argued that a diamond is comprised of many covalently-bonded carbon atoms (therefore being one molecule).Strife wrote:of Carbon-14, each one combines with four other Carbon atoms to make a molecule.
Let he who is without sin cast the first stone...Strife wrote:Thus, several molecules... otherwise you're an idiot
Since when has DNA been visible to the naked eye?Strife wrote:to think a single molecule can be visible to the naked eye(Exception of DNA or something as such).
You are comprised of carbon, yes, and a multitude of other elements. This is not to mention that those elements are not all covalently linked to each other.Danyael wrote:a diamond is carbon that has been under alot of pressure
since i'm carbon does this make me a molecule
I do it because I can
I can because I want to
I want to because you said I couldn't
I can because I want to
I want to because you said I couldn't
-
PLAYER57832
- Posts: 3085
- Joined: Fri Sep 21, 2007 9:17 am
- Gender: Female
- Location: Pennsylvania
Re: Chemistry Question
There is a bit of a technicality here. An atom of an element IS, by definition, also a molecule. So, a Carbon atom is a Carbon molecule.Balsiefen wrote:It's a giant covalent structure. This, though there is a continuous link between all atoms in it's makeup, is rarely described as a molecule.
However, when you say diamond, you refer to a particular structure. To form that structure takes more than one atom. I have seen references to it as "one" molecule and references to it as groups of molecules. Use the definition your instructors/employer wants.
Not quite. The ocean is a mixture of many elements and compounds, dissolved chemicals like salt, even suspended things like sand. They are not bonded like in a molecule. Diamonds are pure carbon.jonka wrote:no, its not.
that would be like saying the ocean is one molecule.
No, it makes you a lump of compressed coal.Danyael wrote:a diamond is carbon that has been under alot of pressure
since i'm carbon does this make me a molecule
-
Strife
- Posts: 2668
- Joined: Fri Jul 20, 2007 3:24 pm
- Gender: Female
- Location: Now something has kept me here too long.
Re: Chemistry Question
This is just sad. Try google next time you want to say something, that way you know. I can't believe you seriously asked a question and are trying to answer it yourself, and you're giving yourself wrong answers. If you keep up this ignorant attitude you probably won't continue to get help on CC, so don't bother posting questions.
~~~~~
I believe that is wrong player, an atom by definition is not a molecule. This site should explain that quiet well: http://www.astrosociety.org/education/f ... /atom.html. Unless I missed your point and explanation of how you got to that conclusion, and you just proved many scientists wrong.
Superheat is how natural diamonds are formed... along with pressure.LYR wrote:Superheated? That is not how natural diamonds are formed...Strife wrote:There's no such thing as a "pure diamond"... it's just multiple carbon atoms, combined into carbon molecules, compressed and superheated
No, the diamond would most definitely not shatter at all, crack, maybe(pushing it to extreme heights). Shatter, not unless the floor beneath was made of diamonds as well, even so pushing it. If a diamond would break from a simple fall they wouldn't have diamond tipped drills, which drill through nearly anything with the exception of man-made super metals.Since when has a diamond been near indestructible? If I dropped it off of a skyscraper it would shatter into a million pieces. Hard, yes, but indestructible... no.Strife wrote:and several molecules of such combined into chains which make the near indestructible lattice structure of the diamond.
Wrong-o... you seem to be more and more ignorant with each passing sentence I read by you. ~ They're Carbon-Carbon bonds, which is why it is argued that they are made of several molecules, which are made of several atoms.No, a diamond if comprised of much more than "several atoms..."Strife wrote:A diamond is just several atoms
They do not combine with each other, they all share electrons with each other, which is why it can be argued that a diamond is comprised of many covalently-bonded carbon atoms (therefore being one molecule).Strife wrote:of Carbon-14, each one combines with four other Carbon atoms to make a molecule.
Dun, dum, dumb... man, I heard ignorance is bliss.Let he who is without sin cast the first stone...Strife wrote:Thus, several molecules... otherwise you're an idiot
http://en.wikipedia.org/wiki/Molecule. "Oh the macro-molecule, me didn't know that is a molecule. heheAHHAHAH!"Since when has DNA been visible to the naked eye?Strife wrote:to think a single molecule can be visible to the naked eye(Exception of DNA or something as such).
~~~~~
I believe that is wrong player, an atom by definition is not a molecule. This site should explain that quiet well: http://www.astrosociety.org/education/f ... /atom.html. Unless I missed your point and explanation of how you got to that conclusion, and you just proved many scientists wrong.
Re: Chemistry Question
Strife wrote:I believe that is wrong player, an atom by definition is not a molecule. This site should explain that quiet well: http://www.astrosociety.org/education/f ... /atom.html. Unless I missed your point and explanation of how you got to that conclusion, and you just proved many scientists wrong.
Website wrote:A molecule is a combination of two or more atoms bonded together. For example, a molecule of water (designated by the symbol H2O) consists of two hydrogen atoms and an oxygen atom which are in a "relationship" — held together by an electric attraction.
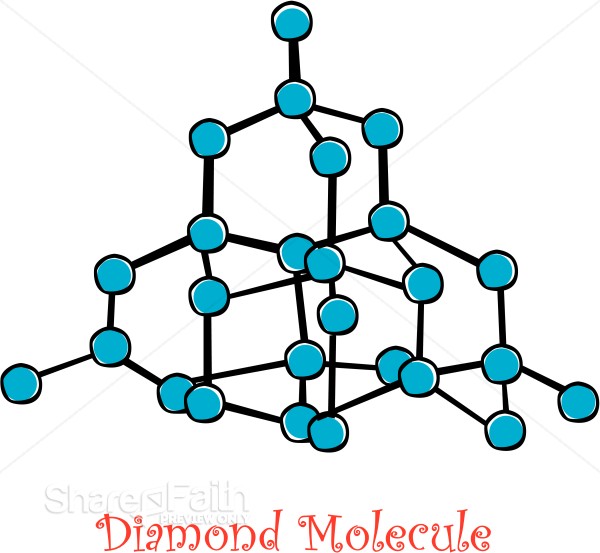
How does that not describe diamond?Diamond Structure wrote:
natty_dread wrote:Do ponies have sex?
(proud member of the Occasionally Wrongly Banned)Army of GOD wrote:the term heterosexual is offensive. I prefer to be called "normal"
Re: Chemistry Question
Yes they are, water molecules are polar.john9blue wrote:The molecules of water aren't linked though. It's just a bunch of H20's, with some NaCl and more contaminants.jonka wrote:no, its not.
that would be like saying the ocean is one molecule.
-
PLAYER57832
- Posts: 3085
- Joined: Fri Sep 21, 2007 9:17 am
- Gender: Female
- Location: Pennsylvania
Re: Chemistry Question
That definition was in an old textbook I had (ironically enough that hit the ashcan shortly after... lol). Actually, I have heard it said that it takes 2 atoms to make a molecule. I am not a chemist and won't argue the point.Strife wrote: I believe that is wrong player, an atom by definition is not a molecule. This site should explain that quiet well: http://www.astrosociety.org/education/f ... /atom.html. Unless I missed your point and explanation of how you got to that conclusion, and you just proved many scientists wrong.
The bottom line is that a diamond is bound by loose bonds and not usually considered a molecule.